© 2023 ANACON RESEARCH PVT. LTD. , Designed By SNT Solutions
ClINICAL RESEARCH
- Home
- ClINICAL RESEARCH
About Clinical Research

• End to end management of clinical trials (Phase I to Phase IV) with accurate Planning, Execution and Clinical data management.
• End-to-end BE study (in NHV & Patients) management for complex generics.
• CRO Pre-qualification Audits
• Monitoring of Bioequivalence studies & Clinical Phase I to IV trials globally
• Clinical study design, review of Protocol, CRF, ICF, CSR, Statistical analysis/Randomization
• Monitoring of Medical devise trials globally
Risk Based Monitoring For Clinical And Bio-analytical Phase
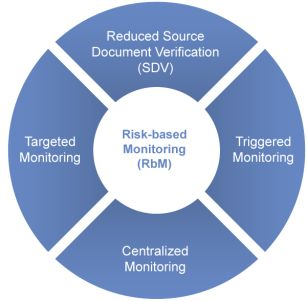
- Compliance with In-house SOP’s, Study specific protocol, GCP/GLP/GDP standards & Specific regulatory standards are the key areas of focus during monitoring.
- Clinical/Medical expert doctors with sufficient experience to advise and guide as per needs.
Clinical Phase Monitoring
- Pre-study documentation and feasibility check - Site Initiation Visit (SIV)
- Monitoring of ongoing study activities & documentation check - Interim Monitoring Visits (Periodic)
- Post study documents compilation and CSR review - Site Close Out Visit (SCV)
Bio-analytical Phase Monitoring
- During study sample/batch analysis ongoing – In-process Bio-analytical Monitoring Visit (IPBA)
- Retrospective review of documents like Method development and validation, all raw data, checks performed at each level, compilation of Bio-analytical report etc..
- Retrospective Bio - analytical Monitoring Visit (RTBA)
CRO Qualification Audits & study specific Audits
Audit is an important element to ensure process, system, instruments, personnel etc…in advance to
verify compliance with GCP and regulatory specific guidance.

Qualification Audit of each and every components from facility to study conduct.
Complete review of Quality Management System and its level of Implementation.
Periodic routine audits to ensure compliance and continuous improvements.
Audit to check on reproducibility of all study related activities and review of study dataretrospectively to ensure compliance with GCP, SOP, Protocol & Regulatory guidance. It isconducted before Inspection by Regulatory Authority.


